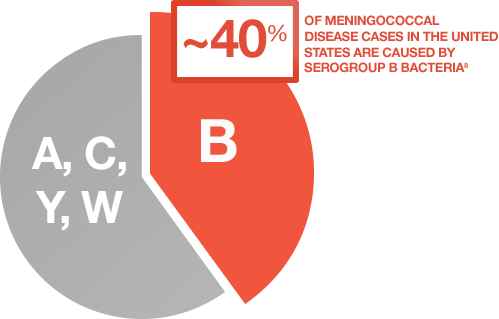
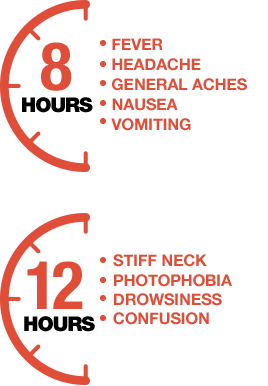
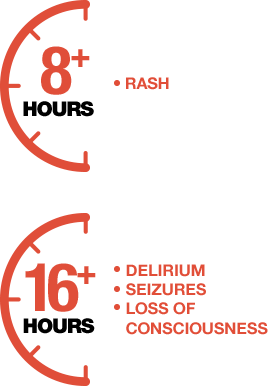
References: 1. Lucidarme J, Comanducci M, Findlow J, et al. Characterization of fHbp, nhba (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B meningococcal ST269 clonal complex isolates from England and Wales. J Clin Microbiol. 2009;47(11):3577-3585. 2. Racloz VN, Luiz SJD. The elusive meningococcal meningitis serogroup: a systematic review of serogroup B epidemiology. BMC Infect Dis. 2010;10:175. 3. Borg J, Christie D, Coen PG, Booy R, Viner RM. Outcomes of meningococcal disease in adolescence: prospective, matched-cohort study. Pediatrics. 2009;123:e502-e509. 4. Poland GA. Prevention of meningococcal disease: current use of polysaccharide and conjugate vaccines. Clin Infect Dis. 2010;50(suppl 2):S45-S53. 5. Cohn AC, MacNeil JR, Harrison LH, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50(2):184-191. 6. McNeil LK, Zagursky RJ, Lin SL, et al. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol Mol Biol Rev. 2013;77(2):234-252. 7. Christensen H, May M, Bowen L, et al. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853-861. 8. Centers for Disease Control and Prevention (CDC). Summary of Notifiable Diseases—United States, 2013. MMWR. 2013;62(51-52):719-732. 9. Meningitis Research Foundation. Meningococcal meningitis and septicaemia: guidance notes: diagnosis and treatment in general practice, 2014 edition. http//. Accessed September 29, 2014. 10. Thompson MJ, Ninis N, Perera R, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367(9508):397-403. 11. Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2013;62(RR-2):1-28.